Estimating nitrogen mineralization: an example
If you’ve got some organic matter in the soil, then you will get some nitrogen (N) that the grass can use from it. This is called mineralization. As the organic matter decomposes, some of the N that was in the organic matter is converted into inorganic forms in the soil.
You don’t get all the N that is in the soil OM all at once. Do you ever see all the soil organic matter decompose in one year? Of course not. What you can expect is from 1 to 4% of the soil organic matter to decompose each year.1 And on average soil organic matter has about 5% N in it.
This makes it straightforward to calculate approximately how much N you may get from a soil with a known amount of organic matter.2 For example, in temperate regions, you can expect about 2 g N/m2 (0.4 lbs N/1000 ft2) to be mineralized in a year for every 1% of soil organic matter in the top 10 cm (4 inches). Is that meaningful where you are? Maybe. But this is a crude approach, because it is not considering site temperatures. This 1 to 4% method works to get a general idea.
For ATC soil test reports, I use site temperatures and the site’s soil organic matter test results to predict monthly and annual totals.3
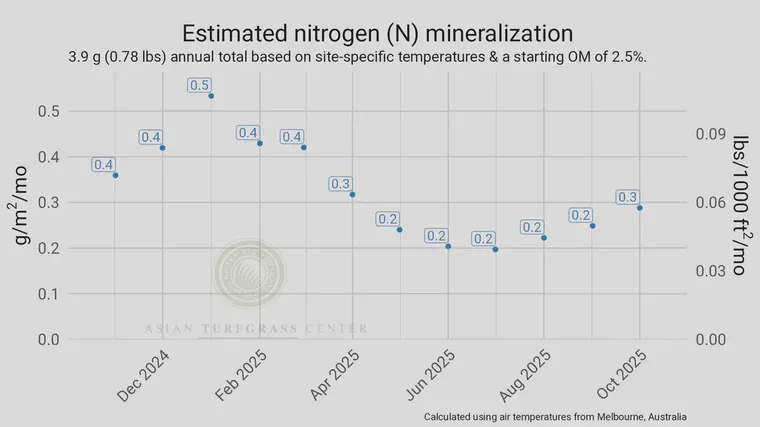
I made the chart above based on a conversation last week about mineralization. The site is in Melbourne, Australia, has soil test data to a 0–5 cm (2 inch depth), and the average soil organic matter in the top 5 cm was 3.5%.
How did I go about making the estimate of N mineralization?
First, I wanted to calculate this for a 10 cm depth, not a 5 cm depth. Based on ATC’s soil test database, if the soil organic matter in the top 0–5 cm of the rootzone is 3.5%, the soil organic matter for the full 0–10 cm depth will be about 2.5%. A sand rootzone with an organic matter content of 2.5% is expected to have a bulk density of about 1.35 g/cm3.
If I calculate the 1% and 4% amounts, that gives me an expected upper and lower bound. And then let’s see how that compares to the 3.9 g annual total predicted by the daily calculation method I used for the chart above.
One square meter of a putting green to a depth of 10 cm with a bulk density of 1.35 will have a mass of 135 kg. The organic matter is 2.5% of that, so the OM mass is 3,375 g. We can expect 5% of that OM to be N, so we have 169 g of N in that one square meter to a 10 cm depth.
If 1% of the N mineralizes in a year, that would be 1.7 g of N per m2. If 4% of the N mineralizes in a year, that would be 6.8 g.
The chart above, calculated daily (then summed monthly and annually) using the site temperatures and assuming the soil water content is kept at half of field capacity on average through the year, predicts that the greatest amount of N will be mineralized in January, the lowest amount will be mineralized in July, and the annual total will be 3.9 g (or about 0.8 lbs/1000 ft2) in the upcoming year.
Notice that the 3.9 g falls nicely between the minimum estimate of 1.7 and the maximum estimate of 6.8.
The amount of decomposition depends largely on temperature, assuming that the soil has a moderate amount of water in it. In cold places, the decomposition may be close to 1%. In tropical places, maybe even more than 4%. ↩︎
A little more detail about this in my presentations from the OGCSA fall meeting in 2019. ↩︎
Read more about the method I use, based on an article by Gilmour and Mauromoustakos, in this post. ↩︎